11. CORPUSCULAR QUANTUM MECHANICS
New physics tenders
instead of a wave quantum mechanics a corpuscular quantum mechanics. The
physical basis its simple: since all free bodies move on a screw trajectory or
on selfcontained orbit, if they are connected, the screw motion gives particles
all observed wave properties. Quantized data by new physics is explained toа
that the exchange of energy in a microcosmos takes place by exchange of
photons. For any photons the angular momentum is identical and is peer ![]() . Naturally, that half of
photon or its some part can not receive participations in interplays, since a
photon - integral particle, though in some cases can be disintegrated on
component about what will be said in the chapter dedicated properties of a
photon.
. Naturally, that half of
photon or its some part can not receive participations in interplays, since a
photon - integral particle, though in some cases can be disintegrated on
component about what will be said in the chapter dedicated properties of a
photon.
Some examples of applying of a corpuscular quantum mechanics and matching of its outcomes with outcomes of a wave quantum mechanics are adduced below. That not unnerve of the reader by the boring mathematical calculations with them is possible to be acquainted in my monography Уthe Fundamentals of new physics and pictures of universe╗ on a site http://www.new-physics.narod.ru.
Atom of Hydrogenium.
In the chapter лthe universal potential energy of repulsing╗ is obtained the formula for radius of orbit of an electron in a hydrogen-like atom (with one orbital electron):
r0=(mea2)/(Ze2) (1) and for electron-binding energy with a nucleus:
E (tie. e.)=-(Ze2)2/(2mea2) (2).
At absorption a photon
with definite energy (resonant photon) the electron passes from orbit of the
Bohr on more high-altitude orbit. The photon thus fades - its energy is spent
for transition, and angular momentum of a photon, naturally to vanish can not.
It is gained by an electron and on new orbit its angular momentum will be 2![]() . If the electron
in time 10-8 seconds will have no time to return back, and the atom
again will assimilate a photon, the electron will take a higher circular orbit
with a angular momentum 3
. If the electron
in time 10-8 seconds will have no time to return back, and the atom
again will assimilate a photon, the electron will take a higher circular orbit
with a angular momentum 3![]() etc. In whole such behavior will be completely
described by the theory of the Bohr. The formula (1) and (2) coincide the
theory of the Bohr and wave quantum mechanics, specially, if a right member (1)
to multiply and to section into electronic mass and to paste aliquot value
etc. In whole such behavior will be completely
described by the theory of the Bohr. The formula (1) and (2) coincide the
theory of the Bohr and wave quantum mechanics, specially, if a right member (1)
to multiply and to section into electronic mass and to paste aliquot value ![]() :
:
r0=n2×![]() 2/me×Ze2 (3). After these manipulations
targetting to customize expression of an official quantum mechanics under the
theory of the Bohr in a denominator there is an electronic mass. On common
sense it should be in numerator, as in (1), since the electron inertia cannot
be neglected. The orthodox now kick of the writer byа that in mesonic atoms
orbital radius of atoms much less and also corresponds to formula (3). The
writer responds on it: as for all microparticles the angular momentum is
identical:
2/me×Ze2 (3). After these manipulations
targetting to customize expression of an official quantum mechanics under the
theory of the Bohr in a denominator there is an electronic mass. On common
sense it should be in numerator, as in (1), since the electron inertia cannot
be neglected. The orthodox now kick of the writer byа that in mesonic atoms
orbital radius of atoms much less and also corresponds to formula (3). The
writer responds on it: as for all microparticles the angular momentum is
identical: ![]() =miViri,
at increase of mass in k of time, a=Viri
will be diminished too in k of time, but, as in numerator (1) a in the second degree, radius of orbit will be diminished
in as much time. Therefore formula (3) though basically is erratic, gives exact
numerical outcome. All on what we reasoned above has relation to so-called rydberg
atoms. Their creation has become possible only with occurrence of lasers with
high intensity of photon bundles. Thus the atom has time to assimilate a
following photon by not losing angular momentum previous. Therefore levels of
energy of atoms of the Bohr and official quantum mechanics are inspissated in a
direction from a nucleus is rydberg atoms. If such atoms to let alone, the
electron will begin by jumps to be reset, losing each time angular momentum
=miViri,
at increase of mass in k of time, a=Viri
will be diminished too in k of time, but, as in numerator (1) a in the second degree, radius of orbit will be diminished
in as much time. Therefore formula (3) though basically is erratic, gives exact
numerical outcome. All on what we reasoned above has relation to so-called rydberg
atoms. Their creation has become possible only with occurrence of lasers with
high intensity of photon bundles. Thus the atom has time to assimilate a
following photon by not losing angular momentum previous. Therefore levels of
energy of atoms of the Bohr and official quantum mechanics are inspissated in a
direction from a nucleus is rydberg atoms. If such atoms to let alone, the
electron will begin by jumps to be reset, losing each time angular momentum ![]() , and atom will
beam photons carrying away this angular momentum. Therefore number of photons,
absorbed atom should in accuracy equal to number of radiated photons at
returning of an electron into orbit of the Bohr, differently will be broken a
law preservations of angular momentum. Naturally, that neither Bohr, nor
fathers of a quantum mechanics the notions about lasers had not, therefore have
elaborated the particular theory not suitable for broad applying.
, and atom will
beam photons carrying away this angular momentum. Therefore number of photons,
absorbed atom should in accuracy equal to number of radiated photons at
returning of an electron into orbit of the Bohr, differently will be broken a
law preservations of angular momentum. Naturally, that neither Bohr, nor
fathers of a quantum mechanics the notions about lasers had not, therefore have
elaborated the particular theory not suitable for broad applying.
New physics, nothing having against existence of rydberg atoms tenders the scheme, more adequate to experiments. The motion of an electron near to a nucleus is shown on a figure 1.
ааааааааааааааааааааааааааааааааа
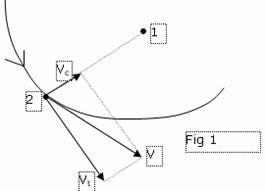
The electron in a point 2 is on some spacing interval from a nucleus arranged in a point 1 and has speed V. is decomposable this speed on two orthogonally related component Vt and Vc. If there is no component speed, directional to a nucleus (Vc), the theory of this case is shown in the chapter лUniversal potential energy of repulsing╗ and coincides the theory of the Bohr and official theory of atom of Hydrogenium. But the presence of component speed Vc in essence changes all picture. Now in a coupling equation of an electron with a nucleus it is necessary to add positive kinetic energy of centripetal motion.
Etie=-Ze2/r+ma2/2r2+mVc2/2 (4). To reduce empty chatter around of the formula (4), we shall put final conclusions: maximum speed Vc apart of radius of Bohr orbit from a nucleus. Bond energy of an electron with a nucleus is determined by expression:
Etie=-(1-1/n2)×Z2e4/2ma2 (5). All parameters of all orbits of atom of Hydrogenium are shown in table 1, and orbits are shown to scale on a figure 2
Table 1.
|
n |
Orbit |
Bond energy (in E0) |
Space from nucleus at perihelia (in r0) |
Space from nucleus at aphelion (т r0) |
Eccentricity, х |
|
1 |
Lyman |
0 |
1/2 |
¥ |
1 |
|
2 |
Balmer |
3/4 |
2/3 |
2 |
1/2 |
|
3 |
Paschen |
8/9 |
3/4 |
3/2 |
1/3 |
|
4 |
Bracket |
15/16 |
4/5 |
4/3 |
1/4 |
|
а 5 |
Phund |
24/25 |
5/6 |
5/4 |
1/5 |
|
Е |
... |
... |
... |
... |
... |
|
¥ |
Bohr (ground state) |
1 |
1 |
1 |
0 |
|
Relation from n |
|
|
|
|
|
аааааааааааааааааааааааааааааааааааааааааааааааааа
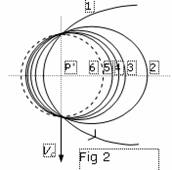
Orbits infinite set and all of them are inspissated about orbit of the Bohr. On a figure 2: 1 - orbit of the Lyman (parabolic), 2 Ц orbit of the Balmer, 3 Ц orbit of the Paschen, 4 Ц orbit of the Bracket, 5 Ц orbit of the Phund, 6 - orbit of the Bohr. As 2 sizes of atom are visible from a figure are augmented with increase of a quantum number unsignificantly. It is a little critics of the official scheme of energy levels of Hydrogenium, introduced on a figure 3.
ааааааааааааааааааааааааааааааааа
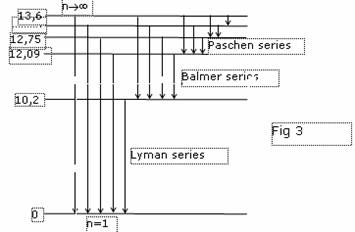
To throw an electron on the first excited level, the energy 10.2 eV is necessary (there correspond to temperature some thousand degrees). But if inflated by Hydrogenium a rubber bead and to take it in arms, we shall fix heat radiation of Hydrogenium. This fact speaks that the levels of energy near to a ground state of atom of Hydrogenium so are close, that the small effect suffices to displace an electron on one of these levels. At opposite transition we shall receive the applicable radiation. About this speaks also heat radiation ambient us bodies. If we shall irradiate Hydrogenium with photons with energy 10.2 eV, in a series of аLyman we shall find out only one line, instead of the infinite set, as it is watched in a spectrum. Under the official theory the sizes of atom of Hydrogenium are augmented proportionally to square of a main quantum number. Inside the Sun consisting, basically, from Hydrogenium temperature is quite sufficient for excitation of atoms up to quantum number 100 and even more. Thus the size them should be increased in 10000 times. Then radius of the Sun in 47 times will exceed spacing interval up to the Earth and will burn down all grief - theorists with the theories. The formula for шчьхэхэш\ of energy of atom at transition into fixed orbit is simple: DE=13.605×e2, where e - eccentricity of orbit. The system, tendered new physics, of energy levels of atom of Hydrogenium is shown on a figure 4.
ааааааааааааааааааааааааааааааааааа
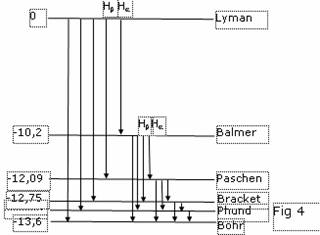
On it we shall finish arguing the theory of atom of Hydrogenium, since understandable physics should be brief physics.